Synthetic transformation of hydroxymatairesinol from Norway spruce (Picea abies) to 7-hydroxysecoisolariciresinol, (+)-lariciresinol and (+)-cyclolariciresinol
Abstract
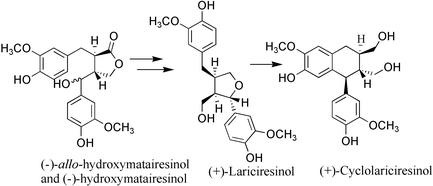
We have developed a method for the transformation of hydroxymatairesinol to optically pure (+)-lariciresinol and (+)-cyclolariciresinol via the hitherto unreported lignan 7-hydroxysecoisolariciresinol. The two naturally occurring isomers of hydroxymatairesinol were reduced with LiAlH4 to a mixture of two epimers of 7-hydroxysecoisolariciresinol, which were further selectively transformed to (+)-lariciresinol and (+)-cyclolariciresinol by an acid catalysed intramolecular cyclisation reaction. The structure of the major isomer of 7-hydroxysecoisolariciresinol was confirmed by

 Please wait while we load your content...
Please wait while we load your content...