Synthesis and lipase catalysed stereoselective acylation of some 3-methylalkan-2-ols, identified as sex pheromone precursors in females of pine sawfly species
Abstract
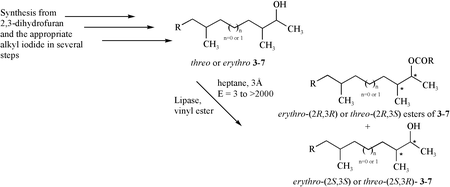
Several 3-methylalkan-2-ols precursors to sex pheromones of Diprion pini, Gilpinia pallida, Gilpinia frutetorum, Diprion nipponica, Macrodiprion nemoralis and Microdiprion pallipes were synthesised as stereoisomeric mixtures in moderate to good yields. The key reaction sequence in the syntheses was the ring opening of either cis- or racemic trans-epoxybutane using a higher order cyanocuprate as nucleophile followed by a highly efficient lipase catalysed stereoselective acylation of the obtained 3-methylalkan-2-ol. The biologically active species specific stereoisomer was synthesised as a single stereoisomer in high stereoisomeric purity, as one in a mixture of two or as one of four stereoisomers when the appropriate 3-methylalkan-2-ol was stereoselectively acylated using a Pseudomonas sp. lipase as catalyst.

 Please wait while we load your content...
Please wait while we load your content...