The use of π-allyltricarbonyliron lactone complexes in the synthesis of the resorcylic macrolides α- and β-zearalenol
Abstract
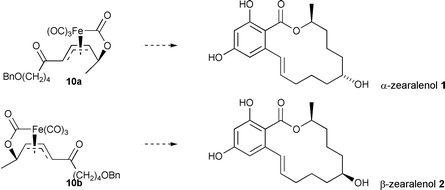
A highly stereoselective synthesis of the natural products α- and β-zearalenol 1 and 2 has been achieved using π-allyltricarbonyliron lactone complexes to control the 1,5-stereochemical relationship of the oxygen functionalities found in these resorcylic

 Please wait while we load your content...
Please wait while we load your content...