Approaches to the synthesis of (2R,3S)-2-hydroxymethylpyrrolidin-3-ol (CYB-3) and its C(3) epimer: a cautionary tale
Abstract
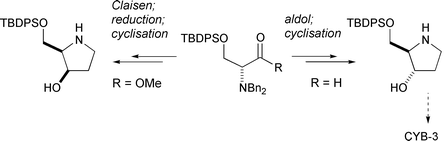
The syntheses of (2R,3S)-2-tert-butyldiphenylsilyloxymethylpyrrolidin-3-ol (TBDPS-protected CYB-3) (21) and its C(3) epimer (25) have been achieved in 9 and 8 steps respectively from D-serine. However, chiral HPLC analysis of the key β-hydroxy ester intermediates in these syntheses (17 and 18) revealed that appreciable levels of racemisation had occurred in the aldol and Claisen condensation reactions used in this synthetic sequence.

 Please wait while we load your content...
Please wait while we load your content...