Exploitation of chemical predisposition in synthesis: an approach to the manzamenones
Abstract
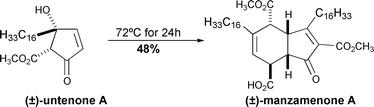
Full details of the syntheses of manzamenones A, C and F are reported, using an approach modelled on a plausible biogenetic theory. The key step of the approach is a “one-pot” conversion of the antileukemic cyclopentenone, untenone A, to manzamenone A which occurs in reasonable yield and which proceeds via a reaction sequence of dehydration, Diels–Alder dimerisation and retro-Dieckmann reaction. The synthetic approach has also been applied to the preparation of a number of shorter alkyl chain analogues of the natural products. Using a combination of NMR and X-ray crystallographic data for the shorter alkyl chain analogues of manzamenone A, it is suggested that the relative stereostructures of the majority of the manzamenones should be revised such that the acyl group at the C2 position lies on the α-face and that at the C5 position resides on the β-face.

 Please wait while we load your content...
Please wait while we load your content...