Extremely simple and practical synthesis of (±)-vertinolide via the Michael addition
Abstract
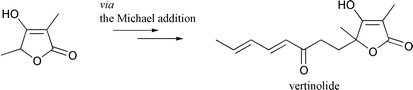
(±)-Vertinolide (1) was synthesized from the readily available tetronic acid derivative 5 in 3 steps. The Michael reaction of the anion derived from 6 with (E)-5-ethoxyocta-1,6-dien-3-one (12) gave the vertinolide precursor 9 in high yield, which was then easily converted to (±)-vertinolide (1).

 Please wait while we load your content...
Please wait while we load your content...