Photochemical transformations of 5-halogeno-4-thiouridines
Abstract
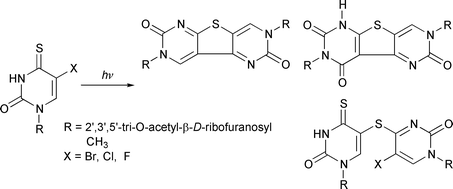
A series of 2′,3′,5′-tri-O-acetyl-5-halogeno-4-thiouridines, where halogen = Br, Cl, F, are synthesized and their photochemical transformations upon irradiation with near-UV light (λ > 300 nm) in aqueous acetonitrile solutions investigated. The main photochemical pathways in each case involve intermolecular additions leading to three main photoproducts with different relative distributions. The photoproducts are isolated and their structures determined based on MS, 1H and 13C NMR and UV spectral data.

 Please wait while we load your content...
Please wait while we load your content...