Preparation of novel O-sulfated amino acid building blocks with improved acid stability for Fmoc-based solid-phase peptide synthesis
Abstract
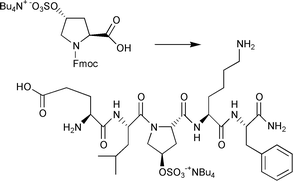
The chemical synthesis and use of O-sulfated hydroxy amino acids in solid-phase peptide synthesis has long been a difficult and delicate task for peptide chemists due to the intrinsic acid lability of the O-sulfate linkage. In this report, a significantly improved method for the introduction and acid-stabilization of sulfate groups onto serine, threonine, and hydroxyproline residues is described. In all three cases, the optimal sulfation conditions were found to be 5 equivalents of sulfur trioxide–N,N-dimethylformamide (SO3·DMF) complex in DMF under anhydrous conditions. The addition of tetrabutylammonium (TBA) counter-ions during work-up served as a powerful O-sulfate stabilization agent. The preparation of Fmoc-Ser(SO3−N+Bu4)-OH 3, Fmoc-Thr(SO3−N+Bu4)-OH 4 and Fmoc-Hyp(SO3−N+Bu4)-OH 6 building blocks gave stable pure products with good solubilities in organic solvents in reproducible, high yields. Importantly, the tetrabutylammonium salts of O-sulfated hydroxy amino acids minimized the desulfation during fluoren-9-ylmethoxycarbonyl (Fmoc)-based peptide synthesis, TFA cleavage, and reversed-phase HPLC purification. Stability experiments with 95% TFA at room temperature showed that for all three derivatives desulfation was less than 5% after standard times for peptide deprotection and resin-cleavage times. In contrast to previous approaches that usually involve the use of sodium and barium salts, the synthesis and mass spectrometric analysis of sulfated amino acids and sulfate peptides was much improved by the presence of tetrabutylammonium salts.

 Please wait while we load your content...
Please wait while we load your content...