Probing the mechanism of loss of carbon-20 in gibberellin biosynthesis. Synthesis of gibberellin 3α,20-hemiacetal and 19,20-lactol analogues and their metabolism by a recombinant GA 20-oxidase
Abstract
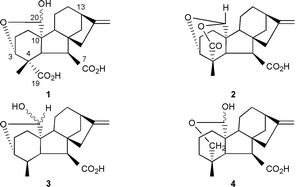
A gibberellin 3α,20-hemiacetal, 1 (equivalent to 3-epi-gibberellin A36), has been synthesised from gibberellin A13. Turnover of this hemiacetal by recombinant gibberellin 20-oxidase occurred with loss of the 20-carbon atom to give the 20-nor-19,10-lactone (3-epi-gibberellin A4). In addition, two other enzyme products were detected and identified by synthesis as 3-epi-20-norgibberellin A13 and 3-epi-1,10-didehydro-20-norgibberellin A13. These by-products indicate that the enzymatic reaction proceeds via a C-10 radical intermediate. Carbon radicals at C-10 and acyl radicals at C-20 were generated chemically and their decomposition products were studied with reference to the biological mechanism. The corresponding 19-nor-3α,20-hemiacetal 3 and 19-methylene analogue 4 were synthesised but were not oxidised at C-20 by the enzyme. These results indicate that the 19-carboxylic acid is an essential component of the enzyme reaction.

 Please wait while we load your content...
Please wait while we load your content...