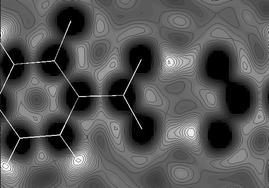
Variable temperature single crystal neutron diffraction has been used to examine the behaviour of protons in the structure of acetylsalicylic acid (Aspirin). The neutron diffraction study, at seven temperatures between 20 and 300 K, has allowed a full description of the molecular structure and the intermolecular interactions in the material and their variation with temperature. In particular the variable temperature studies have allowed the full description of the apparent torsional motions of the terminal methyl group, which exhibit characteristic substantial zero-point motion. The extracted values for the barrier height for the cosine potential governing this motion are found to be in good agreement with that found from ab initio calculations and are compared with those found in a range of other materials. Our previously postulated empirical method for correcting bond lengths in such a case is once again found to have some validity. The data also yield a full description of the various hydrogen bonds present, notably the direct observation of likely anharmonicity in the potential governing the hydrogen bond in the carboxylic acid dimer motif. The availability of data at various temperatures is found to be important in identifying this effect, and in discriminating between alternative explanations of the observed structural parameters. The direct images of protons in various hydrogen bonding potentials, calculated from the neutron data, are also found to be valuable in this context.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...