Synthetic and structural chemistry of enantiomerically pure 1-phenyl-2-carboxyethylphosphonic acid and its derivatives†
Abstract
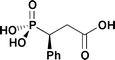
(S)-(+)-1-Phenyl-2-carboxyethylphosphonic acid, (S)-1, has been prepared via diastereoselective alkylation of (3aR,7aR)-octahydro-1,2,3-tribenzyl-1,3,2-benzodiazaphosphole 2-oxide (3) using tert-butyl bromoacetate; the X-ray crystal structure of the intermediate alkylation product is described. The X-ray structure of a single crystal of (R)-1, obtained by crystallisation of rac-1 is reported. The structure of (R)-1 shows a three-dimensional pattern of intermolecular hydrogen bonding involving P(O)⋯HO–C, P–OH⋯(O)C and P(O)⋯HO–P interactions, but without any association between pairs of carboxyl groups. Attempts to resolve rac-1 with various enantiomerically pure bases are reported. The X-ray structure of the hydrated double salt (R)-1-phenylethylammonium rac-1-phenyl-2-carboxyethylphosphonate hydrate is reported; this double salt crystallised unexpectedly from aqueous solutions of the product from rac-1 and rac-1-phenylethylamine.

 Please wait while we load your content...
Please wait while we load your content...