Electrochemical oxidation of La2CuO4 in organic media: influence of the electrolyte composition
Abstract
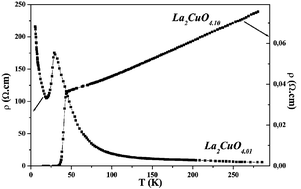
The electrochemical intercalation of oxygen into La2CuO4 has been carried out in various anhydrous organic media: a variety of combinations of dimethyl sulfoxide (DMSO), dimethylformamide (DMF) and acetonitrile (AN) as solvents, NaClO4 or nBu4NBF4 as supporting electrolytes, and the presence or absence of oxygen sources (H2O, KO2) have been studied. The various experiments show that the intercalated oxygen comes mainly from the solvent (DMSO, DMF), which plays a major role, leading, via its decomposition, to the formation of oxygen species able to oxidise the material. Anodic polarisation performed at room temperature gives rise to interesting materials with compositions up to La2CuO4.10, for which superconducting behaviour is observed below 42 K. The physical characterisations of the material (XRD, chemical composition, electronic properties) before and after controlled anodic polarisation are reported. This “chimie douce”-type intercalation proves to be as efficient as that previously performed in alkaline solution.

 Please wait while we load your content...
Please wait while we load your content...