Abstract
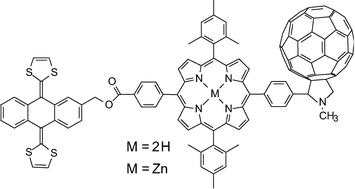
Two molecular triads consisting of a porphyrin (P) covalently linked to a fullerene electron acceptor (C60) and a π-extended tetrathiafulvalene electron donor (TTF) have been synthesized. Time resolved spectroscopic investigations of the triad featuring a free base porphyrin moiety (TTF–P2H–C60) show that in 2-methyltetrahydrofuran solution, excitation of the porphyrin leads to formation of a TTF–P2H˙+–C60˙− charge-separated state in 25 ps. Electron transfer from the TTF generates a final TTF˙+–P2H–C60˙−state with an overall yield of 0.87. This species decays to the ground state in 1.07 µs. Similar experiments on the zinc analog, TTF–PZn–C60, show formation of TTF–PZn˙+–C60˙− in 1.5 ps, followed by generation of TTF˙+–PZn–C60˙− with a yield of 0.09. This charge-separated state also decays to the ground state in 1.07 µs. Comparison of these results with those for previously reported triads with different donor moieties reveals differences in electron transfer rate constants that can be qualitatively understood in the framework of the Marcus–Hush electron transfer formalism.
- This article is part of the themed collection: Functionalised Fullerenes

 Please wait while we load your content...
Please wait while we load your content...