Abstract
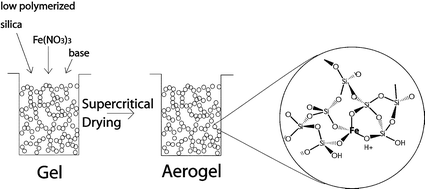
Iron oxide aerogels were synthesized from tetramethoxysilicon(IV) (TMOS) or tetraethoxysilicon(IV) (TEOS) and iron nitrate using an acid-catalyzed solution–sol–gel method combined with subsequent extraction of the alcoholic solvent with supercritical CO2. The main parameters varied in the sol–gel synthesis were: the type of N-base used as the gelation agent (N,N-diethylaniline, trihexylamine, ammonium carbonate, ammonia), the concentration of the iron precursor, and the water content. The silicon precursor was prehydrolyzed to improve its reactivity. After calcination at 600 °C, the structural and chemical properties of the aerogels containing 0–20wt% nominal Fe2O3 were characterized by means of nitrogen adsorption, X-ray diffraction (XRD), transmission and scanning electron microscopy, temperature programmed reduction, X-ray photoelectron spectroscopy (XPS), UV-Vis, DRIFT and EPR spectroscopy. XRD and electron microscopy indicated that all aerogels were amorphous, irrespective of the sol–gel conditions used. The aerogels were predominantly mesoporous, with pore size maxima ranging between 20–50 nm, but also exhibited some microporosity. For the 10 wt% iron oxide samples, the specific pore volumes ranged from 0.7 to 2 cm3 g−1 and BET-surface areas from 150 to 636 m2 g−1, depending on conditions. With increasing iron content, the BET surface area decreased from 740 to 340 m2 g−1, accompanied by increasing microporosity. XPS revealed significant silicon enrichment on the surface. Spectroscopic investigations (UV-Vis, EPR) uncovered different iron-containing species, ranging from tetrahedrally coordinated iron atoms incorporated in the silica matrix to iron oxide nanoclusters. Formation of isolated iron atoms was favored with low iron content samples. The N-base used to force gelation had a significant effect on the morphology and population density of Fe(OH)Si in the aerogels.

 Please wait while we load your content...
Please wait while we load your content...