Abstract
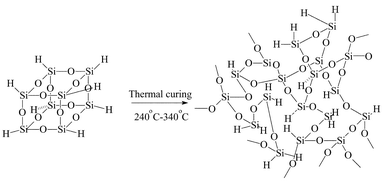
The structures and properties of hydrogen silsesquioxane (HSQ) films produced by curing were studied in the temperature range of 240–340 °C for various curing times. The experimental results show that the transformation of the cage structure to the network structure is the major reaction in the studied temperature range. The cage–network transformation can be explained by two-stage zero order kinetics. The rate constant of the first stage is 10–35 times more than that of the second stage. The

 Please wait while we load your content...
Please wait while we load your content...