Hydroformylation of 1-hexene in supercritical carbon dioxide using a heterogeneous rhodium catalyst. 3. Evaluation of solvent effects
Abstract
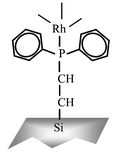
The heterogeneously catalyzed hydroformylation of 1-hexene in supercritical carbon dioxide is demonstrated as an alternative to homogeneous catalysis through the use of a rhodium–phosphine catalyst tethered to a silica support. Reaction over the heterogeneous catalyst in supercritical CO2 is compared with the use of this catalyst in liquid-phase toluene, and toluene expanded with CO2. Likewise, the performance of the tethered catalyst is compared with a homogeneous rhodium–phosphine catalyst, and shown to be equally effective under identical reaction conditions. Comparable reaction rates were obtained using the heterogeneous rhodium catalyst in supercritical CO2 and CO2-expanded toluene, both of which were superior to the reaction rate with the heterogeneous catalyst in liquid-phase toluene. Initial aldehyde selectivity obtained with the heterogeneous species was also comparable to that obtained with the homogeneous catalyst, but decreased over the course of the reaction. These results demonstrate the value of using phase behavior, and the importance of understanding this behavior in the development and analysis of greener solvent/catalyst systems.

 Please wait while we load your content...
Please wait while we load your content...