Eco-friendly synthesis of p-nitrobenzonitrile by heterogeneously catalysed gas phase ammoxidation
Abstract
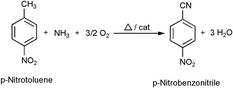
Ammoxidation of p-nitrotoluene (PNT) to p-nitrobenzonitrile (PNB) has been carried out over some bulk and supported vanadium phosphate (VPO) catalysts and also on supported V2O5 catalysts for the first time. The solid PNT was successfully metered over the catalyst bed using a saturator by maintaining the temperature in the saturator above the melting point of PNT and with simultaneous bubbling of a known amount of nitrogen gas as a carrier to the PNT vapours. PNT has been observed to undergo some kind of thermal decomposition at temperatures higher than 623 K even in the absence of catalyst as was evidenced from blank tests. Hence all the catalytic runs had to be restricted to a reaction temperature of 623 K and below. A maximum PNB selectivity of 77% was obtained over a bulk VPO catalyst with a P/V ratio of 1.2 at a conversion of 13% with a high carbon balance of 100%. The lowest PNB selectivity and the carbon balance were given by the catalyst with the lowest P/V ratio (0.5 P/V). The high non-selectivity of this catalyst can be explained on the basis that it contains excess of highly active lattice oxygen and also due to less stabilisation of reduced vanadium species because of the presence of less phosphorus content in the catalyst. In general, the lower activities and selectivities obtained over the studied catalysts have been attributed to the presence of the strongly deactivating –NO2 group on the aromatic ring.

 Please wait while we load your content...
Please wait while we load your content...