Methanesulfonate and p-toluenesulfonate salts of the N-methyl-N-alkylpyrrolidinium and quaternary ammonium cations: novel low cost ionic liquids
Abstract
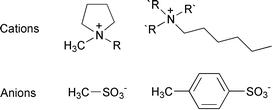
The preparation and characterization of a series of novel salts, based on the N-methyl-N-alkylpyrrolidinium or quaternary ammonium organic cations coupled with sulfonate type anions, namely the mesylate (CH3SO3−) and tosylate (CH3C6H4SO3−) anions are reported. These salts are analogues of the previously described organic cation bis(trifluoromethanesulfonyl)amide (TFSA) salts that form useful ionic liquids of interest in “Green” synthesis. Several of the salts are liquid below 50 °C, e.g. tributylhexylammonium tosylate and ethylmethylpyrrolidinium mesylate and one is liquid at and below room temperature (tributylhexylammonium mesylate). These new salts have a cost advantage over salts of the TFSA−, PF6− and CF3SO3− anions. Electrochemical and thermal properties have been investigated. The salts are stable to beyond 100 °C and exhibit electrochemical potential windows of at least ±2 V vs. Ag/Ag+. Some of the salts exhibit multiple crystalline phases below their melting points, potentially indicative of plastic crystal behaviour, whilst others showed more simple solid–liquid behaviour. Many of the salts were found to be glass forming.

 Please wait while we load your content...
Please wait while we load your content...