The oxidation of alcohols in substituted imidazolium ionic liquids using ruthenium catalysts
Abstract
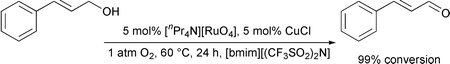
It has been demonstrated that ionic liquids based upon substituted imidazolium cations may be used as alternative solvent media for the selective oxidation of alcohols to aldehydes and ketones. The ruthenium catalyst tetrapropylammonium perruthenate has been used in conjunction with either N-methylmorpholine-N′-oxide or molecular oxygen as oxidants in two different catalytic systems. Benzylic alcohols are oxidized in good to excellent yields whereas aliphatic alcohols require far greater reaction times and give poor yields. The reaction products can be easily removed from the reaction mixture by extraction with diethyl ether.

 Please wait while we load your content...
Please wait while we load your content...