Bis-carbene complexes from oxidative addition of imidazolium C–H bonds to palladium(0)
Abstract
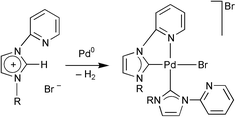
Oxidative addition of the C–H bond at the 2-position of N-(2-pyridyl)imidazolium salts takes place to palladium(0) to lead to unexpected final products containing two carbenes. The formation of either cis or trans bis-carbene complexes, both identified by X-ray crystallography and spectroscopy, apparently is a consequence of the substitution pattern of the imidazolium moiety. A mechanism is suggested based on recent theoretical studies.

 Please wait while we load your content...
Please wait while we load your content...