Variable-pressure kinetic study of bromide complexation on aquarhodium(iii)†
Abstract
A variable-pressure kinetic study of the complexation of bromide with Rh3+(aq)
(I
= 2.03 mol dm−3
(NaClO4−), range 0.1–206.8 MPa) as a function of [H+]
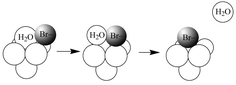
(0.06–1.00 mol dm−3) at 70 °C is reported. The final product is trans-[Rh(OH2)4Br2]+ with formation of the bromopentaaqua ion rate determining. The decrease in the extent of rate saturation with [Br−] observed on lowering [H+] below 0.20 mol dm−3 is consistent with greater participation from the more labile pentaaquahydroxo ion in a parallel ion-pair interchange processes with the hexaaqua ion but with Kos
(RhOH2+–Br−) < Kos
(Rh3+–Br−). Complexation on the hexaaqua ion is associatively activated (ΔV‡I
=
−3.3 ± 1.0 cm3 mol−1) but dissociatively activated on the pentaaquahydroxo species (ΔV‡IOH
=
+7.7 ± 1.0 cm3 mol−1). These findings support the recent ab-initio calculations by Rotzinger et al. and variable-pressure kinetic study of the

 Please wait while we load your content...
Please wait while we load your content...