New pentanuclear mixed valence Co(ii)–Co(iii) complexes of “short” salen homologues†
Abstract
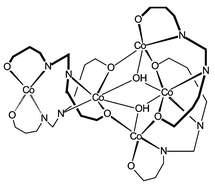
We describe herein a series of novel pentanuclear Co(II)–Co(III) mixed-valence cationic complexes of formula [Co5L4(μ3-OH)2]+ {L2− = 2,2′-[1,1-methanediylbis(nitrilomethylidene)diphenate] or its arylmethane analogues}, obtained by controlled oxidation of [Co2L2] precursors. The [Co5(μ3-OH)2(salNO2ben)4]I·2(CH3)2CO and [Co5(μ3-OH)2(salClben)4]I·2DMF·CH2Cl2 compounds have been studied by X-ray methods and turn out to be isostructural. The structural data show that the cobalt atoms in the pentanuclear units are bridged by μ-O(phenolato) atoms and μ3-OH groups and approximate three types of co-ordination geometries: tetrahedral, octahedral and trigonal-bipyramidal. The X-ray structure of one proligand, namely 2,2′-[1,1-(4-nitrophenylmethanediyl)bis(nitrilomethylidene)diphenol] is also reported. The χT(T) behaviour of the complex with this ligand has been studied in the 4–300 K range. The data clearly show that ferromagnetic as well as (much weaker) antiferromagnetic exchange interactions occur within the cluster. A simple exchange model has been developed which nicely reproduces the experimental data.

 Please wait while we load your content...
Please wait while we load your content...