Preparation of new diazene complexes of ruthenium and osmium†
Abstract
The hydrazine complexes [Ru(NH2NH2)L5](BPh4)2 1, 8 or [Ru(NH2NH2)L′L4](BPh4)2 2 [L = P(OEt)3, L′
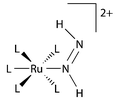
= PPh(OEt)2] were prepared by allowing the corresponding hydride species [RuHL5]BPh4 or [RuHL′L4]BPh4 to react first with HBF4 and then with hydrazine. Oxidation of these hydrazine complexes or [Os(NH2NH2)L5](BPh4)2 with Pb(OAc)4 at −30 °C led to the corresponding stable and isolable 1,2-diazene complexes [M(NH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH)L5](BPh4)2 3, 5 (M =
Ru, Os) or [Ru(NH
NH)L5](BPh4)2 3, 5 (M =
Ru, Os) or [Ru(NH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH)L′L4](BPh4)2 4. The phenyldiazene derivatives [M(PhN
NH)L′L4](BPh4)2 4. The phenyldiazene derivatives [M(PhN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH)L5](BPh4)2 6, 7 (M = Ru, Os) were also prepared by treating the hydride [MHL5]BPh4 species with phenyldiazonium tetrafluoroborate. The aquo-complex, [Ru(H2O){P(OEt)3}5](BPh4)2 8, was obtained by substitution of the NH
NH)L5](BPh4)2 6, 7 (M = Ru, Os) were also prepared by treating the hydride [MHL5]BPh4 species with phenyldiazonium tetrafluoroborate. The aquo-complex, [Ru(H2O){P(OEt)3}5](BPh4)2 8, was obtained by substitution of the NH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH ligand in [Ru(NH
NH ligand in [Ru(NH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH)L5](BPh4)2 and was characterised by X-ray crystal structure determination. Oxidation reactions of the bis(hydrazine) complexes [Ru(NH2NH2)2L4](BPh4)2 or [Ru(CH3NHNH2)2L4](BPh4)2
[L = P(OEt)3] with Pb(OAc)4 were reinvestigated and were found to give the diazene complexes [Ru(NH
NH)L5](BPh4)2 and was characterised by X-ray crystal structure determination. Oxidation reactions of the bis(hydrazine) complexes [Ru(NH2NH2)2L4](BPh4)2 or [Ru(CH3NHNH2)2L4](BPh4)2
[L = P(OEt)3] with Pb(OAc)4 were reinvestigated and were found to give the diazene complexes [Ru(NH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH)2L4](BPh4)2 9, [Ru(CH3N
NH)2L4](BPh4)2 9, [Ru(CH3N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH)2L4](BPh4)2 11 or [Ru(CH3N
NH)2L4](BPh4)2 11 or [Ru(CH3N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH)(CH3NHNH2)L4](BPh4)2 10.
NH)(CH3NHNH2)L4](BPh4)2 10.

 Please wait while we load your content...
Please wait while we load your content...