An unusual dirhenium complex containing the bridging 6-(diphenylphosphino)-2-pyridonate ligand
Abstract
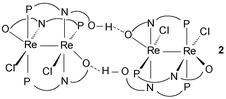
The diamagnetic dirhenium(II) complex Re2Cl2(μ-pyphos)2(pyphosH) (2) is a product of the reactions between (n-Bu4N)2Re2Cl8, Re2(μ-O2CMe)4Cl2 or cis-Re2 (μ-O2CMe)2Cl4(H2O)2 and 6-diphenylphosphino-2-pyridone (pyphosH) in refluxing acetonitrile. This formulation is based upon a single crystal X-ray structure determination and measurements of the magnetic susceptibility, EPR spectrum, electronic absorption spectrum and cyclic voltammetric properties of 2. Unlike the known dimolybdenum(II) complex Mo2(μ-pyphos)4, which contains four N,O-bound pyphos ligands, all three bridging ligands in 2 are N,P-bound. The two anionic pyphos ligands are also involved in weaker ‘axial’ coordination of their O atoms to the dirhenium core. The dirhenium units are linked into dimers-of-dimers through strong intermolecular hydrogen bonds.

 Please wait while we load your content...
Please wait while we load your content...