Hydrocarbonylation reactions using alkylphosphine-containing dendrimers based on a polyhedral oligosilsesquioxane core
Abstract
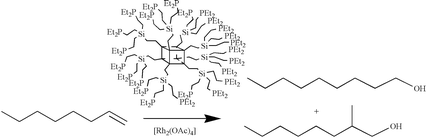
Radical additions of HPR2 (R = Et, Cy) onto alkenyl groups or nucleophilic substitution reactions on chlorosilanes by LiCH2PR2 (R = Me, Hex) are used to prepare first and second-generation alkylphosphine-containing dendrimers based on a polyhedral oligomeric silsesquioxane (POSS) core. The first generation dendrimers (G1) are built on 16 or 24 arms, which are chlorides, vinyl groups or allyl moieties. Hydrosilylation (chlorosilane) followed by vinylation or allylation of octavinyl-functionalised POSS gave these G1 dendrimers. Successive hydrosilylation/allylation followed by hydrosilylation/vinylation produce the framework for the second-generation dendrimer (G2). The phosphorus-containing dendrimers are used as ligands for the hydrocarbonylation of alkenes (hex-1-ene, oct-1-ene, non-1-ene, prop-1-en-2-ol) in polar solvents (ethanol or THF) using the complexes [Rh(acac)(CO)2] or [Rh2(O2CMe)4] as metal source. Linear to branched ratios up to 3 ∶ 1 for the alcohol products are obtained for the diethylphosphine dendrimers. The reactions were found to proceed mainly via the formation of the corresponding aldehydes.

 Please wait while we load your content...
Please wait while we load your content...