Nature of equilibrium shifts in racemic praseodymium(iii) tris(2,2′-oxydiacetate) induced by interaction with chiral probes
Abstract
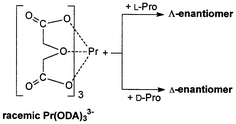
The effects of L-proline and its derivatives N-acetyl-L-proline, L-proline methyl ester, and L-proline benzyl ester on the equilibrium shift in the racemic Pr(ODA)33− complex, in which ODA is 2,2′-oxydiacetate, have been studied by means of circular dichroism (CD) and 1H NMR spectroscopy. While N-acetyl-L-proline did not induce any optical activity in Pr(ODA)33−, the addition of L-proline, L-proline methyl ester, and L-proline benzyl ester led to the appearance of the Pfeiffer effect in this lanthanide complex. The signs of the induced CD signals at 484 nm which correspond to the 3P0 ← 3H4 transition implied that L-proline interacts more favourably with the Λ-enantiomer of the Pr(ODA)33−, while L-proline methyl ester and L-proline benzyl ester preferably interact with the Δ-enantiomer of the same complex. The 1H NMR studies suggested that L-proline and its esters adopt different conformations upon interaction with Pr(ODA)33−. The dependence of the absorption dissymmetry factor, gabs, on the concentration of the L-proline indicated that the Pfeiffer effect in this lanthanide complex is best described by the associated model. Measurements in the solution of various pH, ionic strength, and dielectric constant suggested that the chiral discriminatory interaction is a combination of electrostatic and hydrophobic forces, while the hydrogen bonding effects are less important.

 Please wait while we load your content...
Please wait while we load your content...