Reactivity of fluorinated γ-alumina and β-aluminium(iii) fluoride surfaces towards hydrogen halides and tert-butyl chloride
Abstract
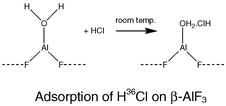
The Lewis acids β-aluminium(III) fluoride and γ-alumina, fluorinated at room temperature with sulfur tetrafluoride, both interact with hydrogen fluoride and chloride, as demonstrated by radiotracer measurements using [18F] and [36Cl]. The different behaviour of HCl towards the two surfaces is rationalised by considering plausible surface sites and, in the case of β-AlF3, the role of residual water. Both materials promote dehydrochlorination of tert-butyl chloride. β-Aluminium(III) fluoride also has some catalytic activity in Friedel–Crafts alkylation whereas oligomerisation of ButCl dominates on fluorinated γ-alumina. The different behaviour appears to be due to the presence of both Lewis and Brønsted surface acidity on γ-alumina that has been fluorinated under static conditions. A description for this surface is proposed.

 Please wait while we load your content...
Please wait while we load your content...