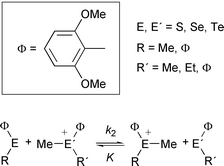
2,6-Dimethoxyphenyl derivatives of sulfur, selenium and tellurium, such as MeΦE, EtΦE, [Me2ΦE]X, [MeEtΦE]X {Φ = 2,6-(MeO)2C6H3; E = S, Se, Te; X = MeSO4, ClO4, or PF6} have been prepared, mostly as crystals. The reaction rates and the equilibrium constants for methyl abstraction by MeΦE from [MeRΦE′]ClO4
{R = Me, Et, Φ; E, E′ = S, Se, Te} were measured by means of 1H NMR spectra. Some representative results include: (1) RΦE (R = Me, Φ; E = S, Se) react commonly with [MeRΦE]+ (R = Me, Et, Φ; E = S, Se) to give the equilibrium mixture, except that MeΦS does not react with [MeEtΦS]+;
(2) RΦE (R = Me, Φ; E = S, Se) do not react with [MeRΦTe]+ (R = Me, Et, Φ); (3) RΦTe (R = Me, Φ) react with [MeRΦE]+ (R = Me, Et, Φ; E = S, Se, Te) to complete the methyl abstraction (E = S, Se) or to give the equilibrium mixture (E = Te); (4) the reaction rate of MeΦE with [MeRΦE′]+ (R = Me, Et, Φ) increases in the orders E = S < Se < Te and E′ = S ∼ Te < Se; (5) reactions of [Me2ΦE]+ are faster than those of [MeEtΦE]+, which react faster than [MeΦ2E]+; (6) Me–E bond strength of [MeΦ2E]+
increases in the order E = S ≤ Se < Te, but those of [Me2ΦE]+ and [MeEtΦE]+ increase in the order E = Se ≤ S < Te; (7) Me–E bond strength of [MeRΦE]+ (E = S, Se) increases in the order R = Φ < Me < Et or Me < Φ < Et, while Me–Te bond strength of [MeRΦTe]+ increases in the order R = Φ < Et < Me.

 Please wait while we load your content...
Please wait while we load your content...