Fluorescent behaviour in host–guest interactions. Part 2.† Thermal and pH-dependent sensing properties of two geometric isomers of fluorescent amino-β-cyclodextrin derivatives
Abstract
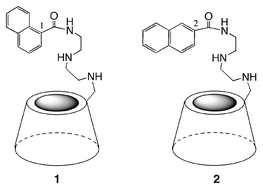
The fluorescent behaviour in aqueous solution of two types of amino-β-cyclodextrin (amino-β-CDx) isomers 1 and 2 bearing the naphthoamide group at the 1- and 2-position as a fluorescent-sensing unit was investigated using mainly

 Please wait while we load your content...
Please wait while we load your content...