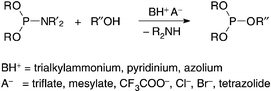
Numerous triflates, mesylates, chlorides, trifluoroacetates and tetrazolides of trialkylammonium, pyridinium and azolium ions have been studied as activators for the reaction of N,N,O,O′-tetraisopropylphosphoramidite with propan-2-ol in acetonitrile. The progress of the reactions was followed by 31P NMR spectroscopy, and the pKa values of the activators were determined by a 13C NMR spectroscopic method based on competing simultaneous protonation of two bases. The salts promoted the reaction both as acids and nucleophiles, the acidity playing a more important role than the nucleophilicity. The Brønsted α value for the general acid catalysis was observed to range from 0.6 to 0.9 and the βnucl
value to be 0.2 (pKa of the conjugate acid used as the measure of nucleophilicity). Mixtures of neutral azoles or pyridines and weakly acidic ammonium salts were also shown to be useful activators that allow the acidity and nucleophilicity to be tuned independently of each other.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...