Competitive occurrence of homolytic N–O and heterolytic C–O bond cleavage in excited-state 1-(arylmethyloxy)-2-pyridones
Abstract
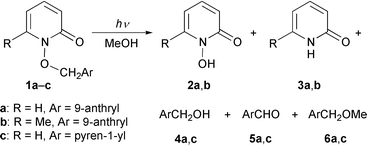
The irradiation at 340 nm of the title compounds having 9-anthryl and pyren-1-yl groups in methanol was found to give the heterolytic C–O bond cleavage products: 1-hydroxy-2-pyridone and aryl-substituted dimethyl ether (which predominate for the reaction of the former title compound) in addition to 2-pyridone, aryl-substituted methanol and aryl-substituted formaldehyde derived from the homolysis of the N–O bond (which mainly occurs in the photolysis of the latter title compound). It was also found that substitution of the methyl group for hydrogen at the 6-position of the pyridone skeleton in 1-(9-anthrylmethyloxy)-2-pyridone decreases the relative composition of the aryl-substituted dimethyl ether to some extent. These substituent effects on the product compositions were explained in terms of stereoelectronic effects on a charge transfer-type interaction between the aromatic and pyridone rings in the singlet excited state. Analyses of the ground-state conformation for the title compounds by MM2 calculations and 1H NMR spectroscopy, as well as of their singlet excited-state behaviour, substantiated the existence of a non-emissive intramolecular exciplex intermediate which plays a key role in inducing the C–O bond heterolysis.

 Please wait while we load your content...
Please wait while we load your content...