Chiral organometallic reagents. Part 27.1 The stereochemistry of the carbomagnesiation of a vinylsilane
Abstract
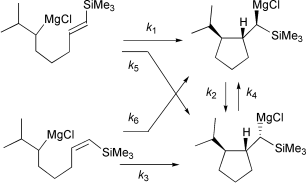
The intramolecular carbomagnesiation of a vinylsilane at 25 °C in THF has been found to proceed in a stereospecific (>95%) syn-addition of carbon and magnesium to the double bond. The resulting α-silylalkylmagnesium compounds are not configurationally stable under the reaction conditions. They epimerize with a half-life of 2.7 d at room temperature.

 Please wait while we load your content...
Please wait while we load your content...