The kinetics of the hydrolysis of aqueous solutions of three sodium C12-alkyl sulfates (SXS), sodium 2-methylundecyl sulfate (SMS), sodium cycloundecylmethyl sulfate (SCS) and sodium 2-pentylheptyl sulfate (SPS), has been investigated at concentrations up to 70% and compared with the behaviour of sodium dodecyl sulfate (SDS). The same kinetic form as previously described for SDS was observed, namely, autocatalysis by protons generated via hydrogen sulfate ion, but there were substantial variations in the reactivity as the alkyl structure changed; β-branching reduced the reactivity, particularly for SMS which was the least reactive of the surfactants studied. The patterns of reactivity by the uncatalysed and hydrogen-ion catalysed pathways for the different SXS were rather similar, but it is argued that the results are consistent with an SN2 mechanism for uncatalysed hydrolysis and the concerted SO3 cleavage (or transfer to a pre-associated water molecule)/proton transfer mechanism for the catalytic route, as previously proposed for SDS. Changes in the microenvironment of the sulfate group in aggregates formed from the different SXS are seen as being responsible for much of the rate variation. Attempts have been made to establish the dependence of observed rate constants in dilute solutions of SXS above the c.m.c. on the water activity as indicated empirically by the rate of pH-independent hydrolysis of 1-benzoyl-3-phenyl-1,2,4-triazole (BPT) in the same solutions. It appears, however, that BPT hydrolysis is not a useful guide to water activity in SXS solutions and values of d(ln k)/d(ln [H2O]) are generally much larger than expected on the basis of simple ideas of transition state composition. The effects of surfactant aggregation on the microenvironment in which chemical reactions take place are suggested to be the dominant kinetic influence both on SXS and BPT hydrolysis.
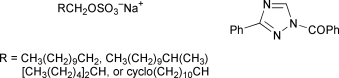
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
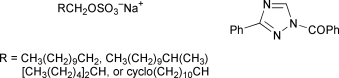

 Please wait while we load your content...
Please wait while we load your content...