Synchronous enantiomeric enrichment of both reactant and product by absolute asymmetric synthesis using circularly polarized light. Part 1.1 Theoretical and experimental verification of the asymmetric photoisomerization of methyl norbornadiene-2-carboxylate to methyl quadricyclane-1-carboxylate†
Abstract
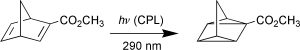
We propose a new absolute asymmetric synthesis (NAAS), in which the irradiation with left- or right-handed circularly polarized light (CPL) of a racemic reactant leads to the synchronous enantiomeric enrichment of both reactant and product. NAAS has two subcategories: (a) reversible NAAS (CPL excites both the reactant and the product), (b) irreversible NAAS (only the reactant is excited by CPL). Here in the first paper of this series of papers we consider irreversible NAAS. We have deduced the theoretical equations that determine the relationship between the enantiomeric excesses (ee's) of both reactant and product and the progress of the CPL-induced photoreaction. Using the clear and reversible photoisomerization of chiral methyl norbornadiene-2-carboxylate (I) to chiral methyl quadricyclane-1-carboxylate (II) by CPL-irradiation in acetonitrile, we experimentally verified the equations. The ee's of both reactant and product are remarkably dependent on the anisotropy factor (g = Δε/ε) of the reactant. The ee of the reactant increases to 100% if the irradiation is continued to the stage that nearly all of the reactant is consumed. Conversely, the ee of the product gradually decreases from g/2 during the initial stages to zero at the final stage of the irradiation. This is the first time that the relationship between the ee of product and the progress of the photoreaction is experimentally examined based upon theoretical considerations.

 Please wait while we load your content...
Please wait while we load your content...