Conformational analysis. Part 36.1 A variable temperature 13C NMR study of conformational equilibria in methyl substituted cycloalkanes
Abstract
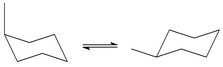
The conformational equilibria in six methylcycloalkanes have been investigated using the temperature dependence of the 13C chemical shifts at temperatures above the coalescence point. Using conformationally homogeneous compounds as standards the temperature dependence was analysed to give the conformer energy differences ΔH (ax–eq). These were for

 Please wait while we load your content...
Please wait while we load your content...