The role of heteroatom substitution in the rigidity and curvature of buckybowls. A theoretical study
Abstract
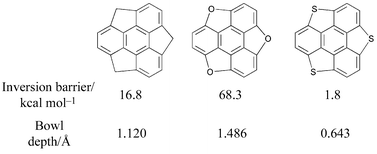
Ab initio (Hartree Fock), hybrid density functional (B3LYP), and semiempirical SCF (MNDO and AM1) calculations on sumanene (2), trioxa-sumanene (3) and trithia-sumanene (4) show that the C3v-bowl structure is a minimum in all cases, but show dramatic variations in bowl depths and inversion barriers. Calculations on monosubstituted corannulenes C19XH10 (X = N+, B−, P+ and Si) at various levels predict that isoelectronic substituents possessing large atomic size increase the bowl-to-bowl inversion barrier at the hub position and decrease it at the rim position. Strain is a guiding factor, which accounts for the relative stability of positional isomers, curvature and bowl rigidity. The most stable positional isomer for a given substituent shows the minimum bowl-to-bowl inversion barrier in all cases. Calculations are performed on monosubstituted sumanenes derived by replacing skeletal C by isoelectronic atoms on sumanene (2), C20XH12 for X = N+ and Si. The general strategy of substituting larger atoms at rim positions flattens the bowl, and at the hub position it makes the bowl deeper. The strategy seems to work well. HF/3-21G and B3LYP/6-31G* computations are in very good agreement with each other, both qualitatively and quantitatively, and the central results are reproducible even at semiempirical levels. The performance of MNDO is consistently better than AM1 and becomes the method of choice when ab initio and DFT methods are not practical. Homodesmic equations, used to ascertain the thermodynamic stabilities of the monosubstitutions on corannulenes and sumanenes, show that substitution at appropriate sites imparts stability to the buckybowl framework. Linear correlation is obtained between the curvature, as estimated by the pyramidalization angle (Φ), and the inversion barrier. It is shown that bowl rigidity, curvature and the relative stabilities of positional isomers are controlled by the strain energy build up, which depends on the size of the substituent and the site of substitution.

 Please wait while we load your content...
Please wait while we load your content...