1,2-Diarylethenes, namely (E )-1-(4-cyanophenyl)-2-phenylethene (1), (E
)-1-(4-cyanophenyl)-2-phenylethene (1), (E )-1-(4-methoxyphenyl)-2-phenylethene (2), (E
)-1-(4-methoxyphenyl)-2-phenylethene (2), (E )-1-(3,4-dimethoxyphenyl)-2-phenylethene (3), (E
)-1-(3,4-dimethoxyphenyl)-2-phenylethene (3), (E )-1-(4-cyanophenyl)-2-(4-methoxyphenyl)ethene (4) and (E
)-1-(4-cyanophenyl)-2-(4-methoxyphenyl)ethene (4) and (E )-1-(4-cyanophenyl)-2-(3,4-dimethoxyphenyl)ethene (5), have been synthesized and their absorption and fluorescence properties at room temperature in different organic solvents and also in 1,4-dioxane–water binary mixtures have been investigated. Additionally, the fluorescence of these compounds has been examined at 77 K in an ethanol–methanol (1
)-1-(4-cyanophenyl)-2-(3,4-dimethoxyphenyl)ethene (5), have been synthesized and their absorption and fluorescence properties at room temperature in different organic solvents and also in 1,4-dioxane–water binary mixtures have been investigated. Additionally, the fluorescence of these compounds has been examined at 77 K in an ethanol–methanol (1 ∶
∶ 1 v/v) matrix. Photophysical parameters like absorption, excitation and fluorescence spectra, fluorescence quantum yields, excited state dipole moment changes, and correlation of solvatochromic fluorescence with solvent parameters like ET(30)-values and the π*-scale have been made. Compound 5, with one cyano and two methoxy substituents, has been found to exhibit solvent polarity-dependent dual fluorescence. The shorter wavelength fluorescence is attributed to an initially excited delocalized planar state, while the longer wavelength fluorescence is attributed to a non-planar twisted intramolecular charge transfer excited state.
1 v/v) matrix. Photophysical parameters like absorption, excitation and fluorescence spectra, fluorescence quantum yields, excited state dipole moment changes, and correlation of solvatochromic fluorescence with solvent parameters like ET(30)-values and the π*-scale have been made. Compound 5, with one cyano and two methoxy substituents, has been found to exhibit solvent polarity-dependent dual fluorescence. The shorter wavelength fluorescence is attributed to an initially excited delocalized planar state, while the longer wavelength fluorescence is attributed to a non-planar twisted intramolecular charge transfer excited state.
 )-1-(4-cyanophenyl)-2-phenylethene (1), (E
)-1-(4-cyanophenyl)-2-phenylethene (1), (E )-1-(4-methoxyphenyl)-2-phenylethene (2), (E
)-1-(4-methoxyphenyl)-2-phenylethene (2), (E )-1-(3,4-dimethoxyphenyl)-2-phenylethene (3), (E
)-1-(3,4-dimethoxyphenyl)-2-phenylethene (3), (E )-1-(4-cyanophenyl)-2-(4-methoxyphenyl)ethene (4) and (E
)-1-(4-cyanophenyl)-2-(4-methoxyphenyl)ethene (4) and (E )-1-(4-cyanophenyl)-2-(3,4-dimethoxyphenyl)ethene (5), have been synthesized and their absorption and fluorescence properties at room temperature in different organic
)-1-(4-cyanophenyl)-2-(3,4-dimethoxyphenyl)ethene (5), have been synthesized and their absorption and fluorescence properties at room temperature in different organic  ∶
∶ 1 v/v)
1 v/v) 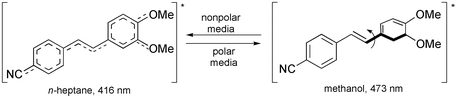

 Please wait while we load your content...
Please wait while we load your content...