Synthesis of the organic ligand of the molybdenum cofactor, in protected form
Abstract
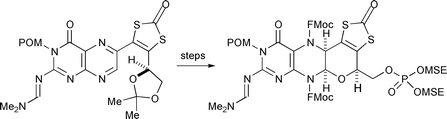
The organic ligand of the cofactor of the oxomolybdoenzymes has been synthesised in the protected and masked form, 5. The key steps in the conversion of the previously prepared 3, a protected 5-(2-amino-4-oxopteridin-6-yl)-4-(1,2-dihydroxyethyl)-1,3-dithiol-2-one were: formation of the pyran ring by reaction with a chloroformate giving a protected 8-amino-4-hydroxymethyl-6-(alkyloxycarbonyl)-5a,6,9,10-tetrahydro-[1,3]dithiolo[4′,5′:4,5]pyrano[3,2-g]pteridine-2,10-dione, 10, cyanoborohydride reduction of the 11,11a-imine, protection at N-11, and finally conversion of the alcohol into a protected phosphate giving 5.

 Please wait while we load your content...
Please wait while we load your content...