Rearrangement of ammonium ylides produced by intramolecular reaction of catalytically generated metal carbenoids. Part 2. Stereoselective synthesis of bicyclic amines
Abstract
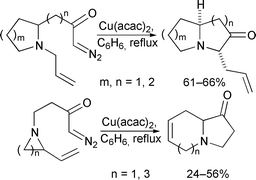
Ammonium ylides produced by intramolecular reaction of copper carbenoids tethered to cyclic allylic amines undergo [2,3]-rearrangement to deliver bicyclic amines. The reaction can be performed using a cyclic N-allylamine in which the diazo group is tethered adjacent to nitrogen, or a vinyl-substituted cyclic amine in which the diazo group is N-tethered to the ring. In the former type of reaction, stereoselective ylide formation and rearrangement usually delivers high levels of diastereocontrol. In the latter case, efficient ‘chirality transfer’ can be accomplished when the reaction is performed using an enantiomerically pure substrate. The reactions have been used to construct pyrrolizidine, indolizidine and quinolizidine systems, and the CE sub-unit found in the manzamine and ircinal alkaloids.

 Please wait while we load your content...
Please wait while we load your content...