Conjugate addition of 6-membered hydrazine to chiral tert-butyl (E)-2-(p-tolylsulfinyl)cinnamates. Synthesis of (S)-celacinnine
Abstract
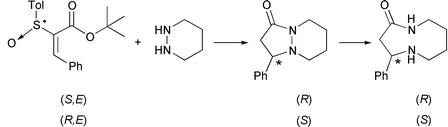
Two enantiomers of the bicyclic lactam, (S)- and (R)-9-phenyl-1,6-diazabicyclo[4.3.0]nonan-7-one (6), were synthesized stereoselectively with high optical purity (95% ee) by the asymmetric conjugate addition–cyclization of piperidazine to chiral vinyl sulfoxides, tert-butyl (E)-2-[(R)- and (S)-p-tolylsulfinyl]cinnamate (4), followed by removal of the p-tolylsulfinyl group with SmI2. The subsequent reductive cleavage of the N–N bond of the bicyclic lactam 6 with sodium in liquid ammonia produced the corresponding 9-membered azalactam, (S)- and (R)-4-phenyl-1,5-diazacyclononan-2-one (7) with 99% and 97% ee, respectively. X-Ray crystallography showed that (S)-7 exists exclusively as a trans conformer in the crystal state. Starting from (S)-7, naturally occurring (S)-celacinnine 1 was synthesized with 99% ee employing the ring-expansion reaction via intramolecular transamidation.

 Please wait while we load your content...
Please wait while we load your content...