Synthesis and study of 4-hydroxymethyl-3-(alkylamino)acridines as models of a new class of DNA-intercalating–alkylating agents
Abstract
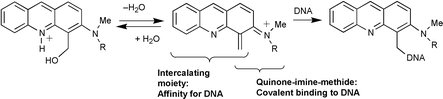
The synthesis, and the reactions with nucleophiles, of 4-hydroxymethyl-3-(dimethylamino)- and -3-(methylamino)-acridines are presented. The reactivity of both compounds in methanol and propan-2-ol is studied. The corresponding 4-methoxy- and 4-isopropoxymethyl-3-(alkylamino)acridines are obtained quantitatively. Kinetics data indicate that protonation of the acridine ring nitrogen greatly increases reaction rates, and results are in favour of a very efficient intramolecular acid–base catalysis generating quinone-imine-methide intermediates. Transetherification reactions (i.e., transformation of methyl ethers into isopropyl ethers) are also observed. The reactivity with DNA is studied. Covalent binding to calf-thymus DNA is evidenced by UV–visible analysis of the modified DNA pellets. Ratios of 1 drug bound per 14 base pairs for the 3-methylamino analogue and 1 drug per 16 base pairs for the dimethyl analogue are calculated, and correspond to 50% of the drugs bound to the macromolecule.

 Please wait while we load your content...
Please wait while we load your content...