First highly stereoselective synthesis of (+)-dihydrosesamin, a trisubstituted tetrahydrofuran-type of lignan, by using highly erythro-selective aldol condensation
Abstract
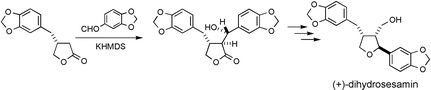
A 2,3,4-tri-substituted tetrahydrofuran-type lignan, (+)-dihydrosesamin 2, was stereoselectively synthesized by using erythro-selective aldol condensation of the potassium enolate from (3R)-3-(3,4-methylenedioxyphenyl)-4-butanolide 3 with piperonal as a key reaction. This is the first stereoselective synthesis of (+)-dihydrosesamin, which is the enantiomer of the natural product.

 Please wait while we load your content...
Please wait while we load your content...