Synthesis and evaluation of a broad range of chiral sulfides for asymmetric sulfur ylide epoxidation of aldehydes
Abstract
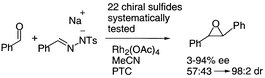
We have recently developed a catalytic, sulfur ylide mediated process for converting aldehydes into epoxides using benzaldehyde tosylhydrazone sodium salt which decomposes to generate phenyldiazomethane in situ. Although chiral 1,3-oxathianes gave good yields and excellent diastereo- and enantio-control when phenyldiazomethane was employed, only low yields were obtained when using the simplified procedure employing benzaldehyde tosylhydrazone sodium salt. Thus, a range of more robust chiral sulfides based on thianes, thiolanes, and 1,4-oxathianes were designed to achieve high yield and high enantioselectivity. The sulfides all possessed the following features: conformationally locked cyclic sulfide in which only one of the two lone pairs was accessible (not relevant for C2 symmetric substrates); ylide conformation and face selectivity was to be controlled through non-bonded steric interactions. Chirality was introduced from chiral pool materials (camphor, amino acids, lactic acid, limonene, carvone, glyceraldehyde), through enzyme mediated reduction/hydrolysis and through the use of chiral reagents (hydroboration). The sulfide catalysts were tested in the reaction between benzaldehyde tosylhydrazone salt and benzaldehyde to give trans-stilbene oxide. The range of chiral sulfide catalysts derived from camphor gave trans-stilbene oxide in generally good yield (23–95%) and with moderate enantioselectivity (40–76% ee). The range of novel chiral thianes and 1,4-oxathianes gave trans-stilbene oxide again in generally good yield (9–92%) and with moderate enantioselectivity (20–77% ee). The range of C2 symmetric chiral sulfide catalysts based on 5 and 6 membered rings gave trans-stilbene oxide in moderate yield (10–78%) and with variable enantioselectivity (8–87% ee). In none of the cases could high enantioselectivity and high yield be achieved simultaneously. Analysis of the results led us to the conclusion that the moderate enantioselectivity was a result of poor control in the ylide conformation and this led to the design of completely rigid [2.2.1] bicyclic sulfides which finally gave high enantioselectivity and high yield in the epoxidation process.

 Please wait while we load your content...
Please wait while we load your content...