Reactions of thiobenzoylketene S,N-acetals with silyl enol ethers of cyclic ketones in the presence of desilylating reagents: formation and desulfurization of thienolactams
Abstract
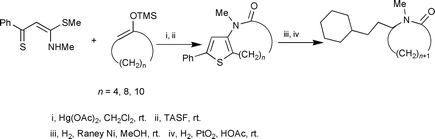
Medium-sized thienolactams can be directly prepared from thiobenzoylketene S,N-acetals, Hg(OAc)2, and silyl enol ethers of cyclic ketones, and either TBAF or TASF. However, by adding either water or alcohol to the foregoing mixture, 3-methylamino-5-phenylthiophenes, in which the ω-position of long-chain alkanoic acids and alkanoic esters are bonded to C-2 of the thiophene ring, can be obtained albeit in low yields. Sequential treatment of the thienolactams with Raney nickel and Adam's catalyst results in completely reductive desulfurization of thienolactam molecules.

 Please wait while we load your content...
Please wait while we load your content...