Synthesis of substituted hexa-3,5-dienoic acid methyl esters from conjugated dienones
Abstract
Substituted hexa-3,5-dienoic acid methyl esters (2) were conveniently prepared in one step by 1,2-carbonyl transposition of the corresponding dienones (1) using
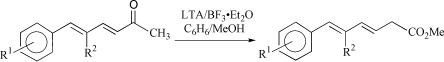

 Please wait while we load your content...
Please wait while we load your content...