Indium-mediated allylation reactions of α-chlorocarbonyl compounds and preparation of allylic epoxides
Abstract
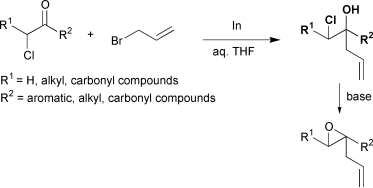
Indium-mediated allylation of α-chlorocarbonyl compounds with various allyl bromides in aqueous media gave the corresponding homoallylic chlorohydrins, which could be transformed into the corresponding

 Please wait while we load your content...
Please wait while we load your content...