Efficient 1,8- and 1,9-asymmetric inductions in the Grignard reaction of δ- and ε-keto esters of 1,1′-binaphthalen-2-ols with an oligoether tether as the 2′-substituent: application to the synthesis of (−)-malyngolide
Abstract
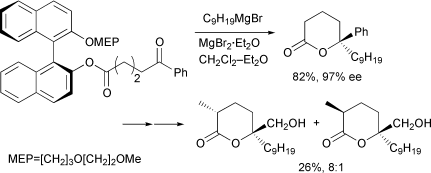
Efficient 1,8- and 1,9-asymmetric inductions in the Grignard reaction of podand-type δ- (3,4) and ε-keto

 Please wait while we load your content...
Please wait while we load your content...