Convenient synthesis of optically active 1,2-diol monosulfonates and terminal epoxidesvia oxazaborolidine-catalyzed asymmetric boranereduction of α-sulfonyloxy ketones
Abstract
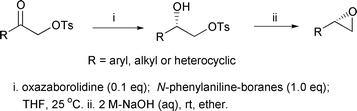
A very convenient asymmetric synthesis of 1,2-diol monosulfonates and terminal

 Please wait while we load your content...
Please wait while we load your content...