Stereocontrol in a one-pot procedure for anionic oxy-Cope rearrangement followed by intramolecular aldol reaction
Abstract
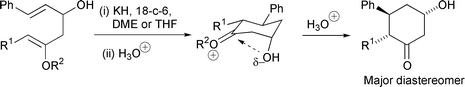
Racemic β-hydroxycyclohexanones with up to three chiral centres have been synthesized in a stereocontrolled way using the novel anionic oxy-Cope (AOC) rearrangement of acyclic enol ethers. The first examples of compounds containing an aldehyde and an enol ether in a 1,5 relationship are reported. Stereocontrol in the cyclisation of these compounds by a 6-(enolendo)-exo-trig intramolecular aldol reaction has been studied: there is high stereoselectivity for an axial hydroxy group in the product β-hydroxycyclohexanones. AM1 calculations show that there is a stabilising electrostatic attraction between the oxygen atom of the axial C-3 hydroxy group and the electron-poor carbon at C-1 in the intermediate oxonium ions. ≥87% of AOC rearrangement occurs via a chair-like transition state giving rise to the 5,6-anti stereochemistry of the β-hydroxycyclohexanones. Trapping the enolate product of AOC rearrangement with oxygen gives fragmentation via a 1,2-dioxetane.

 Please wait while we load your content...
Please wait while we load your content...