Alkali salts (4−) of several 2-substituted alkenylpropanedinitriles are prepared, isolated and characterised by 1H and 13C NMR spectroscopy. By treating the salt 4a− with 4a a dimer, 2-dicyanomethylene-6-methyl-4,6-diphenyl-1,2,5,6-tetrahydronicotinonitrile 6, is formed, for which a single-crystal X-ray structure is presented. Alkylation of the ambident salts 4− with MeX (X = I, Tf, or Br) leads to regioisomers, 1- and 3-alkylation, and comparison with O vs. C-alkylation of ketones is made. Double alkylation is also observed, 3-alkylation followed by 1-alkylation. Thus from 4a− a mixture of (E )- and (Z
)- and (Z )-2-methyl-2-(1-phenylprop-1-enyl)propanedinitrile 9b is formed, and the X-ray structure of the former is presented. Acylation of 4a− with benzoyl chloride gives only the 3-regioisomer; using equimolar amounts of reactants gives a ring-closure product, 2-oxo-4,6-diphenyl-2H-pyran-3-carbonitrile 12, while excess of benzoyl chloride gives the double-acylation product, (Z
)-2-methyl-2-(1-phenylprop-1-enyl)propanedinitrile 9b is formed, and the X-ray structure of the former is presented. Acylation of 4a− with benzoyl chloride gives only the 3-regioisomer; using equimolar amounts of reactants gives a ring-closure product, 2-oxo-4,6-diphenyl-2H-pyran-3-carbonitrile 12, while excess of benzoyl chloride gives the double-acylation product, (Z )-4,4-dicyano-1,3-diphenylbuta-1,3-dienyl benzoate (Z
)-4,4-dicyano-1,3-diphenylbuta-1,3-dienyl benzoate (Z )-13, for which the X-ray structure is presented. Acylation of 4a− with acetyl chloride gives both regioisomers; the 1-acylated product evaded isolation, and the 3-acylation product reacted further to give the (E
)-13, for which the X-ray structure is presented. Acylation of 4a− with acetyl chloride gives both regioisomers; the 1-acylated product evaded isolation, and the 3-acylation product reacted further to give the (E )- and (Z
)- and (Z )-form of 4,4-dicyano-1-methyl-3-phenylbuta-1,3-dienyl acetate 15. The X-ray structure of the latter isomer, (Z
)-form of 4,4-dicyano-1-methyl-3-phenylbuta-1,3-dienyl acetate 15. The X-ray structure of the latter isomer, (Z )-15, is presented. Methoxycarbonylation of salts 4− with methyl chloroformate gives both regioisomers, while use of methyl cyanoformate gives only the 3-regioisomer, in addition to a Michael adduct from reaction of the CN− group with protonated salt 4−.
)-15, is presented. Methoxycarbonylation of salts 4− with methyl chloroformate gives both regioisomers, while use of methyl cyanoformate gives only the 3-regioisomer, in addition to a Michael adduct from reaction of the CN− group with protonated salt 4−.
 )- and (Z
)- and (Z )-2-methyl-2-(1-phenylprop-1-enyl)propanedinitrile 9b is formed, and the X-ray structure of the former is presented.
)-2-methyl-2-(1-phenylprop-1-enyl)propanedinitrile 9b is formed, and the X-ray structure of the former is presented.  )-4,4-dicyano-1,3-diphenylbuta-1,3-dienyl benzoate (Z
)-4,4-dicyano-1,3-diphenylbuta-1,3-dienyl benzoate (Z )-13, for which the X-ray structure is presented.
)-13, for which the X-ray structure is presented.  )- and (Z
)- and (Z )-form of
)-form of  )-15, is presented. Methoxycarbonylation of salts 4− with
)-15, is presented. Methoxycarbonylation of salts 4− with 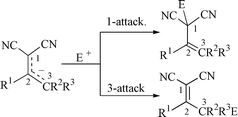

 Please wait while we load your content...
Please wait while we load your content...